Childhood cancer is curable. Modern development in the pharmacotherapy and newer treatment modalities have made it possible to remove the burden of cancer in most cases. This is a series of discussion about chemotherapeutic agents used in the treatment of childhood cancer. Cancer treatment is different from other Paediatric diseases. It needs special care specially infection prevention. Most of the chemotherapy drugs causes bone marrow suppression, ultimately leading to deficiency of bodies protective cell known as neutrophil and monocyte. Some basic knowledge regarding chemotherapy drugs and its side effects are essential to remember even for the parents or caregivers. These post will help to understand the basics of chemotherapy and its drugs for the nurses, medical students, general practitioners and mostly for the residents and students of pediatric hematology and oncology.
Mercaptopurine/Thioguanine
Mercaptopurine and thioguanine are thiol-substituted derivatives of the naturally occurring purine bases hypoxanthine and guanine.
Mercaptopurine has been used in the treatment of ALL, primarily for the maintenance of remission. In standard maintenance regimens, mercaptopurine is administered orally at a dose of 60 to 75 mg/m2/d with upward or downward dose adjustments based on the degree of myelosuppression. when the actual dose of mercaptopurine received increased by 22% as a result of more aggressive
prescribing guidelines, the relapse-free survival improved by 18%.
Thioguanine has been used in the treatment of acute myelocytic leukemia and is administered orally in doses of 75 to 100 mg/m2 daily for 5 to 7 days or in doses of 40 to 60 mg/m2 daily for more prolonged courses. The thiopurines are prodrugs that must be converted intracellularly to thioguanine nucleotides to exert a cytotoxic effect. The active intracellular metabolites are phosphorylated thiopurine nucleotides, which inhibit de novo purine synthesis and purine interconversion and are incorporated into DNA, resulting in inhibition of DNA synthesis and function. The thiopurines also undergo S-methylation, catalyzed by thiopurine methyltransferase or TPMT.
The level of intracellular TPMT activity is an important determinant of the availability of thiopurines for conversion to active thioguanine nucleotides, and, as a result, TPMT regulates the cytotoxic effect of these thiopurines. One in 300 patients is deficient of TPMT activity and extremely sensitive to the cytotoxic effects of mercaptopurine, thioguanine, and azathioprine.
When mercaptopurine is coadministered with the xanthine oxidase inhibitor allopurinol, the fraction of the dose absorbed increases fivefold and a dose reduction of oral mercaptopurine is recommended. Bioavailability was diminished in nonfasting patients and patients experiencing nausea and vomiting. Food decreases the absorption of the drug. Should be taken on empty stomach, one hour before meal at night or at least 2 hour after food taken.
TPMT Deficiency:
TPMT activity test (phenotype)—this method tests the activity level of the enzyme thiopurine S-methyltransferase (TPMT) in a person’s red blood cells.
TPMT genetic test (genotype)—an alternative test to TPMT enzyme activity level is a genetic test that can identify genetic variations in the TPMT gene.
Each person has two copies of the TPMT gene. Most people have two copies of “wild type” TPMT that produce sufficient TPMT enzyme. Approximately 10% of people have one copy of the wild-type gene and one copy of a gene variation associated with decreased TPMT (heterozygous) and intermediate enzyme activity OR little or no enzyme activity (homozygous).
Toxicity:
- Myelosuppression (primary toxic effect of thioguanine)
- Hepatic dysfunction (elevated transaminases, cholestatic jaundice), and
- Mucositis
- Dry skin
- Urticaria
- Photosensitivity
De-oxy-adenosine Analogs: Fludarabine/Clofarabine
De-oxy-guanosine Analogs: Nelarabine
Pyrimidine Antimetabolites-Cytarabine
Cytarabine (cytosine arabinoside, ara-C), an arabinose nucleoside analog of deoxycytidine.
After intracellular metabolic activation, cytarabine interferes with DNA replication and repair through inhibition of DNA polymerase and through incorporation into DNA. Incorporation into DNA inhibits chain elongation, result in chain termination, or cause DNA strand breaks. It only occurs during the DNA synthesis phase (S phase) of the cell cycle, and more prolonged exposure to cytarabine allows the drug to be incorporated into a larger fraction of the cells as they pass through the S phase.
Cytarabine: daunorubicin liposome (CPX-351) is a dual-drug formulation of cytarabine and daunorubicin, packaged at a fixed 5:1 molar ratio within a liposomal carrier. The novel design was developed to optimize both a constant drug ratio of 5:1 found to have the highest proportion of synergy and lowest antagonism as well as to increase drug exposure while minimizing toxicity.

Mechanism of action:
Ara-C is transported into cells mainly via nucleoside transporters including solute carrier family 29 member 1 (SLC29A1). Subsequently, intracellular Ara-C is phosphorylated to Ara-C monophosphate (Ara-CMP) by deoxy-cytidine kinase (dCK), and then to Ara-C diphosphate (Ara-CDP) by cytidine monophosphate kinase 1 (CMPK1), and eventually to its active form Ara-C triphosphate (Ara-CTP) by several nucleoside diphosphate kinases (NDPKs). Ara-CTP competes with deoxycytidine triphosphate (dCTP) for incorporation into DNA and consequently causes cell death by interfering with DNA and RNA synthesis.
Adverse effects of cytarabine
The primary toxicities of cytarabine are myelosuppression, nausea and vomiting, and gastrointestinal mucosal damage.
- Myelosuppression
- Nausea & vomiting
- Gastrointestinal mucosal damage (bowel necrosis)
- Neurotoxicity: acute cerebellar syndrome manifesting 3 to 8 days after initiation of therapy. Nystagmus, ataxia, dysarthria, dysmetria, dysdiadochokinesia, seizures and encephalopathy
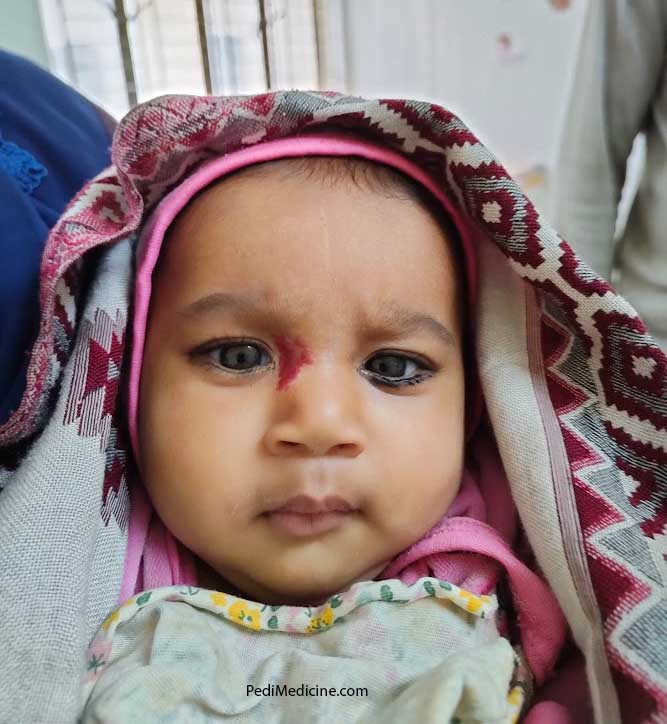
- Ara-c syndrome (fever, myalgia, bone & joint pain, rash, conjunctivitis, chest pain)
- Conjunctivitis and keratitis ( Mx. Steroid eye drop)
- Acute pancreatitis
- Pulmonary complication (ARDS, pulmonary oedema)
Buy cytarabine in Bangladesh Medex
Uracil Analogs: Fluorouracil
Fluorouracil is a prodrug and must be converted intracellularly to nucleotides before expressing cytotoxicity.
The deoxyribonucleotide 5-Fd-UMP is a potent inhibitor of thymidylate synthase, leading to depletion of the DNA precursor, thymidine; and the ribonucleotide FUTP is incorporated into RNA.

Toxicity:
- Myelosuppression
- Mucositis
- Hand-foot syndrome (tingling, numbness, pain, erythema, dryness, pruritis of the hand and feet and desquamation)
- Neurotoxicity (Confusion, Seizure, Cerebellar ataxia)
- Blepharitis
- Acute & chronic conjunctivitis
Here is a quick link to previous topics.
Chemotherapy drugs: Things you need to know
Chemotherapy Drugs for Childhood Cancer Part 2: Basics
Chemotherapy Drugs for Childhood Cancer Part 3 Basics
Chemotherapy Drugs for Childhood Cancer Part 4 Basics
Chemotherapy Drugs Cyclophosphamide Cisplatin Carboplatin
Chemotherapy Drugs for Childhood Cancer Methotrexate
Thanks