Childhood cancer is devastating news to parents. But for better things, the cancer has treatments. Methotrexate was first discovered in 1947, and since then it has been used to treat various types of cancer. It is also used in the treatment of autoimmune diseases. It decreases the body’s immune system. It is often used for the treatment of leukemia, lymphoma, brain tumors, osteosarcoma, etc. Although methotrexate has many early and late adverse effects, its use is sometimes mandatory. It can be prescribed in many forms, like oral, IV, intrathecal, etc. Oral doses should be taken on an empty stomach. Methotrexate is a folate antagonist. It competitively inhibits dihydrofolate reductase. MTX is administered on an intermittent schedule by a variety of routes, including oral, intramuscular, subcutaneous, intrathecal, and IV.
Chronic oral or intramuscular therapy is administered weekly at a dose of 20 mg/m2. IV therapy ranges from a 10-mg bolus to 33,000 mg/m2 as a 24-hour infusion. Doses above 300 mg/m2, which are usually administered by continuous infusion, must be followed by a course of the rescue agent leucovorin (5-formyl-tetrahydrofolate) to prevent the development of severe toxicities.
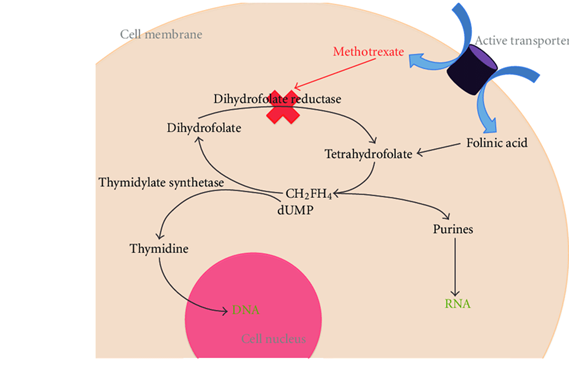
The mechanism by which methotrexate inhibits cellular proliferation. Active transporters include the reduced folate carrier and an endocytic pathway activated by a folate receptor; dUMP: deoxyuridine monophosphate; CH2FH4 : methylenetetrahydrofolate.
Methotrexate is an antimetabolite of the antifolate type. It is thought to affect cancer and rheumatoid arthritis by two different pathways. For cancer, methotrexate competitively inhibits dihydrofolate reductase (DHFR), an enzyme that participates in the tetrahydrofolate synthesis. The affinity of methotrexate for DHFR is about 1000-fold that of dihydrofolate. DHFR catalyzes the conversion of dihydrofolate to the active tetrahydrofolate. Tetrahydrofolate is needed for the de novo synthesis of the nucleoside thymidine, required for DNA synthesis. Also, folate is essential for de novo purine base biosynthesis, so synthesis will be inhibited. Methotrexate, therefore, inhibits the synthesis of DNA, RNA, thymidylates, and proteins.
Infusion durations of up to 42 hours are tolerable when followed by leucovorin rescue. In clinical practice, infusion durations range from 4 to 36 hours depending on the type of cancer being treated. Patients who are treated with an HDMTX infusion must be adequately hydrated and alkalinized to prevent precipitation of MTX in acidic urine, and routine monitoring of urinary output, serum creatinine, and plasma MTX concentrations is mandatory to determine the duration of leucovorin rescue. For most infusion regimens, 12 to 15 mg/m² of leucovorin should be continued every 6 hours until plasma MTX concentration decreases to 0.05 to 0.1 μM. The bioavailability of oral MTX can also be significantly reduced when administered with food. Should be taken 1 hour before or 2 hours after food/on an empty stomach.
MTX is eliminated primarily by renal excretion. HD-MTX should not be given to patients with a creatinine clearance of less than 50% to 75% of normal. Low-dose therapy should be withheld in patients with a serum creatinine level greater than 2 mg/dL.
Toxicity:
The primary toxic effects of MTX are myelosuppression and/or oro-intestinal mucositis, which occur 5 to 14 days after the dose. The development of toxic reactions is related to the concentration of the drug and the duration of exposure.
An early rise in serum creatinine (1.5 times baseline) within the initial 24 hours can help identify a population of patients at increased risk for delayed MTX elimination. Aggressive hydration and alkalinization, as well as increasing the sodium content of the hydration fluids, can prevent drug precipitation and result in enhanced excretion of MTX.
Neurotoxicity from HDMTX includes an acute, stroke-like encephalopathy, seizures, and chronic leukoencephalopathy. Hepatic toxicity consisting of transient elevations of serum transaminase. The development of renal dysfunction during HDMTX is a medical emergency. Glucarpidase, a recombinant bacterial enzyme that catabolizes MTX to the inactive metabolite 4-amino-4-deoxy-N10-methylpteroic acid (DAMPA), rescues patients who develop MTX nephrotoxicity by providing an alternative route of elimination.
Other side effects include a dermatitis characterized by erythema and desquamation, allergic reactions, and acute pneumonitis. MTX osteopathy is a cumulative toxicity that causes bone pain, osteoporosis, and an increased risk of fractures.
Adverse Effects:
- Myelosuppression
- Mucositis
- Nephrotoxicity
- Acute cerebellar dysfunction
- Acute chemical arachnoiditis (headache, nuchal rigidity, seizures, vomiting, fever after intrathecal administration)
Drug Interactions (Drugs avoided during HD-MTX)
The most significant interactions involve agents that interfere with MTX excretion, primarily by competing for renal tubular secretion. These drugs include
- Probenecid
- Salicylates
- Sulfisoxazole
- Penicillins
- Ciprofloxacin
- Nonsteroidal anti-inflammatories indomethacin, ketoprofen, and ibuprofen
- Proton pump inhibitors omeprazole, rabeprazole, and pantoprazole.
- Nephrotoxic drugs, such as the aminoglycosides, vancomycin, and cisplatin, may also alter the clearance of MTX.
Intrathecal Methotrexate
It is administered adjuvant to patients with newly diagnosed ALL to prevent meningeal relapse. An acute chemical arachnoiditis characterized by headache, nuchal rigidity, vomiting, fever, and CSF pleocytosis can present several hours to days after a dose.
An ascending radiculopathy with loss of primarily motor function resembling Guillain–Barré syndrome is also associated with intrathecal MTX, as well as cytarabine, and occurs days to weeks following a course of therapy.
Why is the IT-MTX dose based on age instead of BSA?
In young children, CSF volume increases much more rapidly than does the body surface area, reaching 80% of the adult volume by the age of 3 years. An intrathecal dose based on body surface area would underdose young children and overdose adolescents. Therefore, the intrathecal dosage schedule for MTX is based on age instead of body surface area.
Precaution
- Hydration (starting 12 hours before and 24-48 hours after MTX infusion)
- Alkalization of urine (sodium bicarbonate in IV fluid to ensure that urinary pH > 7, usually up to 24 to 48 hours)
- With high-dose therapy, methotrexate blood levels should be monitored every 24 hours starting at 24 hours after methotrexate infusion.
- Inj. Folinic Acid—start 24 hours after MTX infusion, 6 hourly for 12 doses until plasma conc. is below 0.05–0.1 micM.
- Oral care.
- Regular monitoring of renal and liver function
Bibliography:
- Pizzo and Poplack’s Pediatric Oncology, 8th Edition
The Methotrexate Story Article on Indian Journal of Rheumatology
- MTX Tablet in Bangladesh Medex Sellers website
Impact of Pharmacogenetics on High-Dose Methotrexate Toxicity in Pediatric Oncology
Late effects of high-dose methotrexate treatment in childhood cancer survivors—a systematic review –www.researchgate.net
Here is a quick link to previous topics.
Chemotherapy drugs: Things you need to know
Chemotherapy Drugs for Childhood Cancer Part 2: Basics
Chemotherapy Drugs for Childhood Cancer Part 3 Basics
Chemotherapy Drugs for Childhood Cancer Part 4 Basics
Chemotherapy Drugs Cyclophosphamide Cisplatin Carboplatin
Thanks All.